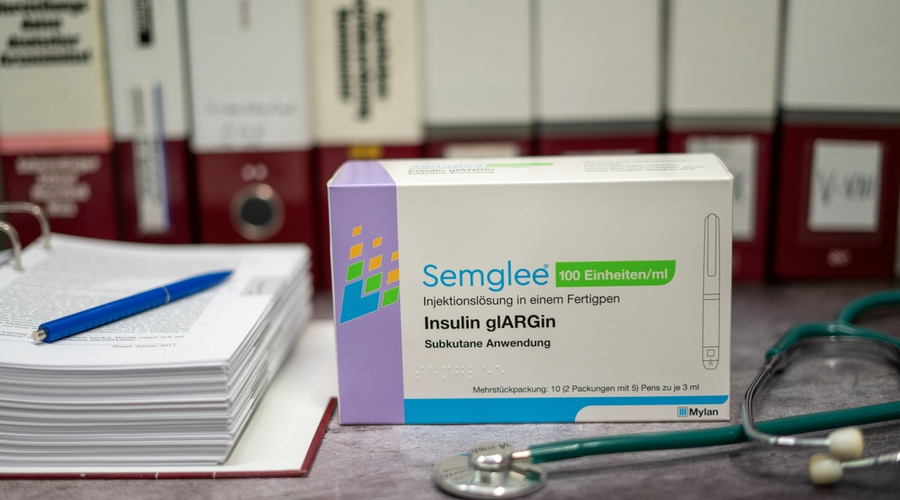
In 2021, the very first interchangeable biosimilar insulin product, Semglee (insulin glargine-yfgn), was approved by the U.S. Food and Drug Administration. It acts as a less costly option to the long-acting insulin Lantus (insulin glargine) for patients with diabetes, and it has been shown to improve glycemic control in adults and children with type 1 diabetes and in adults with type 2 diabetes.
With Semglee now commercially available to retail pharmacies, pharmacists have their first opportunity to dispense an interchangeable biosimilar to their patients. This is a milestone, because the approval process for a biosimilar product has many requirements to prove it is very similar to its reference product.
To be approved as a biosimilar, the company seeking approval must show the FDA that the data are very similar. They also must show mitogenicity, as well as perform large studies with people who have type 1 and 2 diabetes to prove the insulin is highly similar in terms of safety, efficacy, and immunogenicity. These are the FDA standards for biosimilars; interchangeables, however, are a bit different.
For a product to be approved as an interchangeable, the FDA requires that the company holds studies that show they get the same clinical results as the reference product. There cannot be diminished efficacy, signs of safety issues, and absolutely no sign of difference in immunogenicity—and the FDA can change their requirements at any time. With approved products, interchangeability allows pharmacists to automatically substitute an interchangeable biosimilar in place of a reference product, without having to consult a prescribing physician.
For a better understanding on how prepared pharmacists are to accept the opportunities of biosimilars, 115 retail pharmacists were surveyed by Cardinal Health in the 2022 Biosimilars Report. The results have shown that while many say they’re optimistic about biosimilars helping to lower costs for patients, there are still questions and concerns.
While most retail pharmacists have some familiarity with biosimilars and the concept of interchangeability, many have concerns about the FDA-approval process, especially in relation to the use of extrapolation. This information indicates a critical need to improve awareness and understanding of the science behind the FDA approval process for biosimilars so pharmacies can feel confident dispensing them.
While pharmacists are still familiarizing themselves with biosimilars, nearly all respondents agreed they feel comfortable with substituting biosimilars for reference products when the costs are lower for patients. Pharmacists agree lower prices will help patients feel more comfortable taking a biosimilar, but the level of price discount required to motivate pharmacists to recommend biosimilars to their patients is still not clear and may depend on PBMs and insurance companies.
Because pharmacists are the most trusted healthcare professionals in America, education on biosimilars is extremely important. Pharmacists should hold meetings with their team members so that everyone understands the differences between generic, biosimilar, and interchangeability and can explain them to their patients.
To educate patients about biosimilars, pharmacists will need additional educational resources, such as fact sheets on biosimilars. These must be written at the appropriate literacy level that can be shared with their patients. Educating and counseling patients will be an extremely important part of biosimilar adoption, as will making sure healthcare providers and team members alike are equipped with the education. This will make patients feel much more comfortable and accepting of the biosimilar in their healthcare journey.
Looking for great educational resources on biosimilars for medical professionals, patients, or payers? Visit the Biosimilars Council website.
Are biosimilars and generics the same?
While they may have their similarities, don’t be fooled. They’re actually very different. While both are marketed as cheaper versions of expensive name-brand drugs, and both are also designed to have the same clinical effect as the pricier medications, here’s how they differ:
- Generic drugs are identical to the original in chemical composition
- Biosimilar drugs are highly similar, but close enough in duplication to accomplish the same therapeutic and clinical result
- Generics are copies of synthetic drugs
- Biosimilars are modeled after drugs that use living organisms as important ingredients
From the Magazine
This article was published in our quarterly print magazine, which covers relevant topics in greater depth featuring leading experts in the industry. Subscribe to receive the quarterly print issue in your mailbox. All registered independent pharmacies in the U.S. are eligible to receive a free subscription.
More articles from the June 2022 issue:
- Cultivating Your Leadership Style
- Semglee Is Now Available at Retail Pharmacies
- Tackling Burnout in the Pharmacy
- Refresh Your Pharmacy With a Remodel
- Modern Marketing with QR Codes
- Profitable Expansion with Remote Delivery Kiosks
- Choosing the Right Accounting Method
- Measuring and Maximizing Wholesaler Rebates
A Member-Owned Company Serving Independent Pharmacies
PBA Health is dedicated to helping independent pharmacies reach their full potential on the buy-side of their business. Founded and run by pharmacists, PBA Health serves independent pharmacies with group purchasing services, wholesaler contract negotiations, proprietary purchasing tools, and more.
An HDA member, PBA Health operates its own NABP-accredited warehouse with more than 6,000 SKUs, including brands, generics, narcotics CII-CV, cold-storage products, and over-the-counter (OTC) products — offering the lowest prices in the secondary market.