Updated June 15
Performing coronavirus tests is a great opportunity to improve the health of your community and set up your pharmacy as a resource during these unprecedented times. But before you place your order for test kits, educate yourself about the different kinds of tests and regulations that come along with this new guidance. The NCPA has released a helpful webinar on COVID-19 testing, and we’ve distilled information from that webinar, along with research from additional sources, to give you an overview of COVID-19 testing in your pharmacy.
Types of Testing
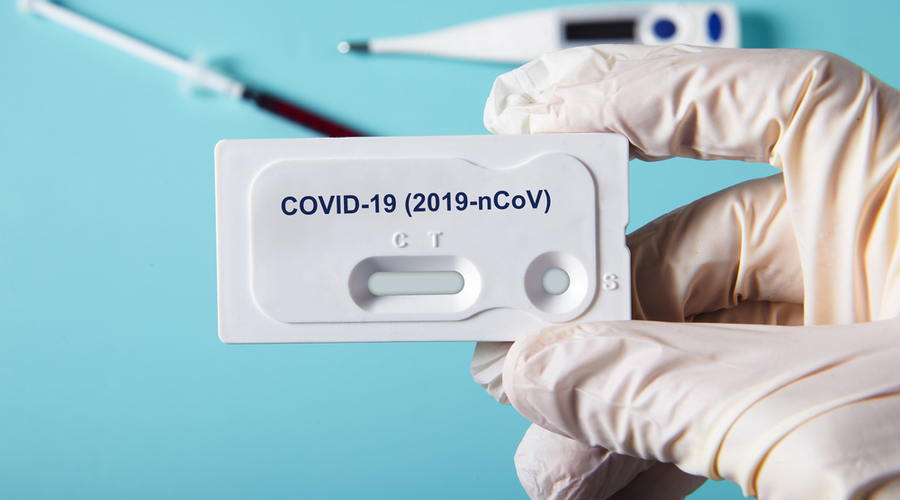
There three ways to test for coronavirus, but not all of them can be easily performed in your pharmacy. They also serve different purposes, depending on whether or not the patient is exhibiting symptoms. Here’s what you need to know about the tests:
- PCR or Molecular Testing: These tests are performed with a nasal or throat swab test, and they are best used before a patient starts exhibiting symptoms or while they are still exhibiting symptoms. Generally, this kind of testing is difficult to perform in a provider office like a pharmacy. However, Walgreens and CVS are using Abbott’s ID NOW™ COVID-19, a molecular test that claims to deliver positive results in as little as five minutes and negative results in 13 minutes. However, on May 14, the FDA issued an alert warning that the Abbott ID NOW test may return false negative results.
- Antigen Testing: Antigen tests are also performed with a nasal or throat swab. They generally can’t detect the virus before patients show symptoms, so they should only be used on patients that are currently symptomatic. It can be easily performed in your pharmacy. Generally, these tests are easier to implement than PCR tests and less expensive. The first antigen test specific to the coronavirus, manufactured by Quidel Corporation, was granted emergency authorization by FDA on May 8. The test can produce diagnostic results within minutes by quickly detecting proteins found on or within the virus. However, with antigen tests, positive results are very accurate, but a negative result does not necessarily rule out the the virus.
- Antibody Testing: Sometimes known as a serology test, antibody tests use a blood sample to look for an immunity response to the virus. This kind of test starts being useful 10 to 14 days after infection, and patients receiving this kind of test should be asymptomatic — either they are over their symptoms or they never had them. Antibody testing is easy to perform at the pharmacy and results are available in 10 to 15 minutes. As of June 29, no COVID-19 serology tests are authorized by FDA for the CLIA-waived point-of-care setting.
For a more detailed explanation of COVID-19 testing methods, see this article from GoodRx.
Before You Test
In this unprecedented situation, things are changing fast. The FDA is making accommodations and exceptions to ensure there is widespread availability of testing across the country, but the amount of information being released can be hard to sift through. Here are the things you need to know about before you decide to start testing for COVID-19 in your pharmacy.
Emergency Use Authorization
During public health emergencies, the FDA can grant Emergency Use Authorizations (EUA) to allow unapproved medical products onto the market to help diagnose, treat, or prevent serious health conditions.
Because of the urgency of the coronavirus pandemic, COVID-19 test kits currently on the market have not been rigorously vetted by the FDA. However, manufacturers have submitted information about their products to the FDA to receive EUA status.
The letter of authorization for the test will describe appropriate testing settings — that means it could specifically or exclude settings like pharmacies. So, while you want to make sure the test kits you purchase have an EUA, not every single EUA test will be suitable for your pharmacy.
To see all the COVID-19 test kits that have been granted an EUA, visit the FDA’s website. Tests that haven’t been granted EUA status are not suitable for point-of-care testing.
CLIA Waivers
Under normal circumstances, non-clinical sites that plan to offer testing are required to obtain a Clinical Laboratory Improvement Amendments (CLIA) Certificate of Waiver. This enables you to perform tests that have been waived under CLIA, which includes all COVID-19 tests with EUA status.
Pharmacies who want to perform COVID-19 testing need to have a CLIA waiver. The form to apply for a waiver can be found here.
Pharmacies Can Enroll as Independent Clinical Diagnostic Laboratories
Pharmacies and other suppliers currently enrolled in Medicare may also enroll temporarily as
independent clinical diagnostic laboratories during the COVID-19 public health emergency.
Pharmacies and other suppliers who are not currently enrolled in Medicare and want to enroll as
an Independent Clinical Diagnostic Laboratory, must submit a CMS-855B enrollment application
to the Medicare Administrative Contractor (MAC) serving your geographic area.
To maintain billing privileges as an Independent Clinical Laboratory, the pharmacy must submit a CMS855B enrollment application within 30 days after the public health emergency ends to the MAC serving your geographic area.
Billing for COVID-19 Testing
Stay updated with this NCPA resource.
According to the NCPA, “Medicare will cover certain COVID-19 tests performed by pharmacists if they are enrolled in Medicare as an Independent Clinical Laboratory, in accordance with scope of practice and state laws. With these changes, beneficiaries can get tested at ‘parking lot’ test sites operated by pharmacies consistent with state requirements.”
Medicare Part B covers clinical diagnostic laboratory tests, including COVID-19 diagnostic tests,
under the Clinical Laboratory Fee Schedule (CLFS), without any beneficiary cost-sharing requirements.
Medicaid may cover COVID-19 tests and laboratory processing of self-collected COVID-19 tests that are FDA-authorized for self-collection.
Fraudulent Tests
With so many people desperate to get their hands on testing supplies, there are unfortunately people out there eager to take advantage of that desperation. The market is currently rife with fraudulent testing kits, and because of that, you need to do your due diligence before you place any orders to make sure the product is legitimate.
The NCPA recommends first making sure that the manufacturer is registered with the FDA. After that, ask the company for legal and clinical validation of the test. If the validation they show you hasn’t been completed using PCR, look for another kit.
To comply with FDA Guidelines, test kits must be accompanied by this language:
- This test has not been reviewed by the FDA.
- Negative results do not rule out SARS-CoV-2 infection, particularly in those who have been in contact with the virus. Follow-up testing with a molecular diagnostic should be considered to rule out infection in these individuals.
- Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status.
- Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E.
As of May 18, the FDA has only authorized two COVID-19 test kit that patients can perform by themselves at home: LabCorp’s “Pixel” and Everlywell’s at-home kit. If you see any other at-home test kits being advertised, that’s a red flag.
Personal Protective Equipment
You’re already on the front lines of the coronavirus pandemic, making contact with potentially ill patients every day. By performing tests, you’re inviting even more risk into the pharmacy.
Before you decide to provide COVID-19 testing, make sure you have enough personal protective equipment on hand to keep you and your staff members safe.
For healthcare personnel evaluating potential COVID-19 patients, the CDC recommends donning an isolation gown, a NIOSH-approved N95 facepiece or higher, face shields or goggles, and gloves that cover the cuff of your gown.
The CDC has guidance for best practices when it comes to using personal protective equipment on its website.
Why Should You Test?
Testing for COVID-19 could put you in harm’s way, and it might be tempting to play it safe. But the argument for pharmacy-based testing is simple: it saves lives.
You are the most accessible healthcare provider for most people, and you can provide much-needed support for your community. In 21 percent of ZIP codes, independent pharmacies are the only pharmacies for patients to seek help in these uncertain times.
Identifying people who have already recovered from COVID-19 can also help those who are still sick. The antibodies in their blood can be transfused into COVID-19 patients who don’t have immunity with a treatment known as human convalescent plasma.
The more widespread testing is, the faster people can return to work and help to restart the economy, improving the situation for your business in the long term. At the moment, the payment model for coronavirus testing is cash only, making it a good financial opportunity for independent pharmacies.
By offering testing, you can also set yourself up as a coronavirus resource center in your community. When a vaccine becomes available sometime next year, you will be the first place people think of to go and get vaccinated.
An Independently Owned Organization Serving Independent Pharmacies
PBA Health is dedicated to helping independent pharmacies reach their full potential on the buy side of their business. The company is a member-owned organization that serves independent pharmacies with group purchasing services, expert contract negotiations, proprietary purchasing tools, distribution services, and more.
PBA Health, an HDA member, operates its own VAWD-certified warehouse with more than 6,000 SKUs, including brands, generics, narcotics CII-CV, cold-storage products, and over-the-counter (OTC) products.
Want more pharmacy business tips and advice? Sign up for our e-newsletter.